Oxygen reduction via the oxygen reduction reaction (ORR) is fundamentally important to many energy conversion and storage technologies such as metal-air batteries and fuel cells. This reaction requires the combination of four electrons and four protons to achieve oxygen reduction, whereby the O=O double bond is broken and two molecules of water are formed. Central to the use of this reaction in technological applications is the development and fundamental understanding of catalysts capable of assisting in this complicated reaction.
O2 + 4H+ + 4e– –> 2H2O ORR
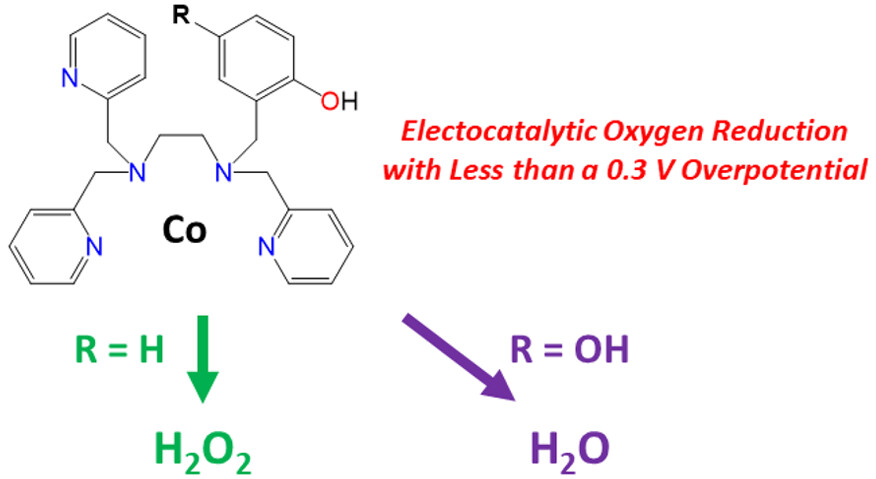
Our research in this area has involved multiple directions, from solid state to molecular catalysts. On the solid state front, we have collaborated with the Comes group to investigate metal oxide catalysts capable of driving ORR (and the reverse reaction OER: oxygen evolution reaction) to better understand their fundamental activity, structure, and stability. Specifically, perovskite and spinel oxide materials are grown synthetically using molecular beam epitaxy (MBE) to produce atomically precise and single crystal thin films (10-50 nm). Our group then studies the electrocatalysis of these materials towards ORR (and OER). On the molecular side, we have collaborated with the Goldsmith group to investigate iron and cobalt catalysts with pendent quinol functional groups which can provide the necessary electron and proton equivalents to achieve efficient oxygen reduction.
Publications related to this work:
- “Installing Quinol Proton/Electron Mediators onto Non-Heme Iron Complexes Enables Them to Electrocatalytically Reduce O2 to H2O at High Rates and Low Overpotentials” Inorg. Chem. 2024 (ASAP)
- “Co(II) Complex with a Covalently Attached Pendent Quinol Selectively Reduces O2 to H2O” J. Am. Chem. Soc. 2022, 144, 22826-22830.
- “Oxygen Reduction Electrocatalysis with Epitaxially Grown Spinel MnFe2O4 and Fe3O4“ ACS Catal. 2022, 12, 3577-3588.
- “Thickness Dependent OER Electrocatalysis of Epitaxial LaFeO3 Thin Films” J. Mater. Chem. A 2022, 10, 1909-1918.